From the ACD, “…the Committee concluded that pegloticase effectively lowers the level of uric acid in the blood for a significant proportion of patients with severe gout. However the Committee noted the risk of serious adverse reactions, and concluded that there was considerable uncertainty about the long-term efficacy and safety of pegloticase. As the cost per quality-adjusted life-year was considered to be over £66,000, which is above the £20-30K range normally considered cost-effective, the committee's provisional recommendation is that pegloticase would not represent a cost-effective treatment option for the NHS.”
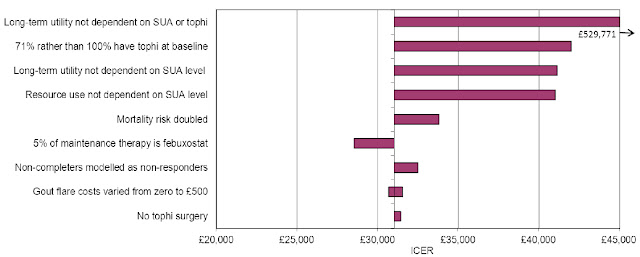
ScHARR-TAG, with HEDS, were the Evidence Review Group for NICE on this appraisal. The team - Rachel Archer, Sarah Davis, Lesley Uttley, Anna Cantrell - identified a number of weaknesses in the manufacturer’s cost-effectiveness analysis, with their exploratory analyses summarised in a remarkably one sided tornado plot (below). Seven amendments to the baseline estimate increased it from around £30,000 per QALY gained to the £67,000 per QALY gained that appears to be highlighted in the ACD.
Source: ERG Report